SASRI is pioneering a promising solution to tackle one of the industry’s most persistent pest problems – the dreaded stalk borer, eldana. This solution involves genetically modified (GM) sugarcane engineered with CRY proteins, nature’s own insecticides produced by soil-borne bacteria. This solution forms one arm of an integrated pest management approach.
Traditionally, SASRI has evaluated the resistance of these GM sugarcane varieties to eldana through pot trials, a process that demands mature stalks and extensive time. Unfortunately, this method proved impractical for assessing and comparing the resistance of engineered cane during the early stages of their development in the GM programme.
Enter the In Vitro Plantlet Bioassay (IVPB), a cutting-edge innovation developed by SASRI. This revolutionary approach allows the assessment of GM sugarcane resistance to eldana larvae without the lengthy wait.



The five-day bioassay has resulted in the development of an eldana resistance rating scale that allows the ranking of GM cane according to their resistance to the pest. The assay considers plant damage as well as larval weight and mortality.
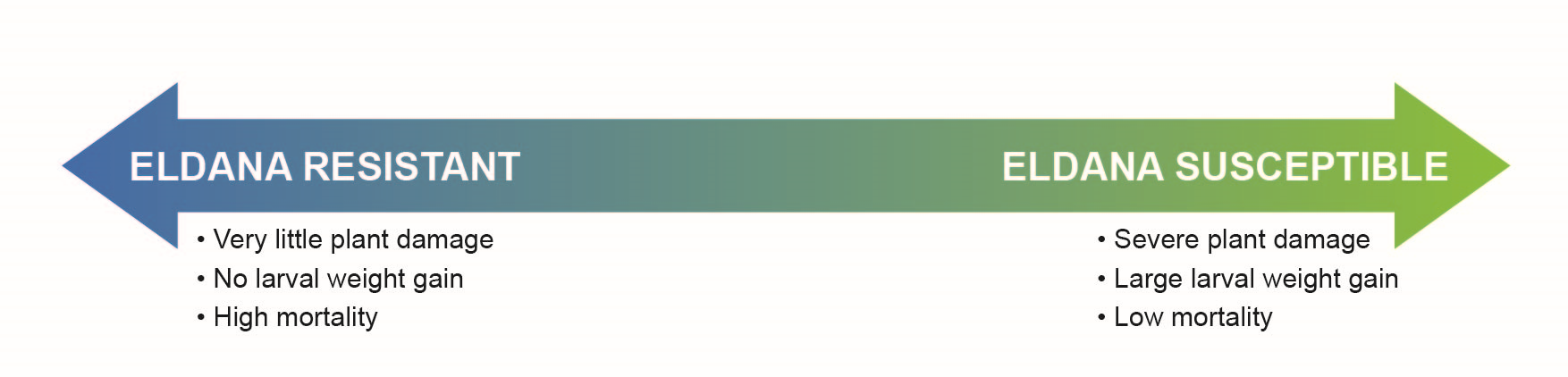
The result?
The IVPB is more than just a time-saver; it’s a game-changer for SASRI’s GM programme. Instead of the traditional two-year timeline for pot trial assessment, this new method cuts the time required down to less than six months.
This not only expedites the GM programme but also enables the early identification of superior GM plantlets, contributing to greater efficiency and improved resource utilisation.

