Variety Improvement
Key Areas
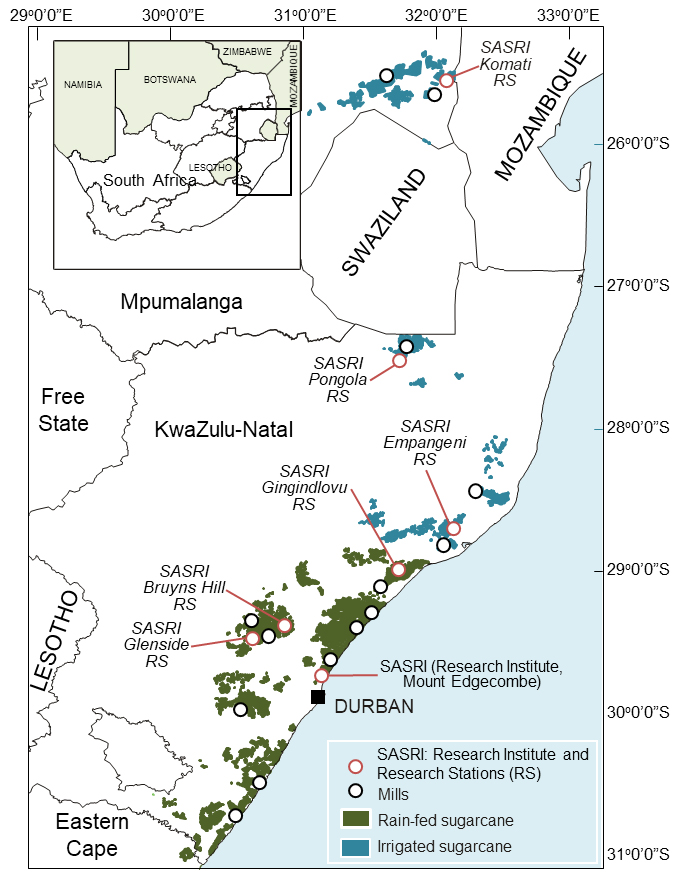
This programme conducts research and implements strategies for the continual release of high yielding, adaptable, pest and disease resistant varieties that add value and enhance industry productivity. Supporting the programme are six research stations strategically sited throughout the SA sugar belt, which are operated by highly skilled technical and farm support staff.
Commercial Breeding lies at the core of the Variety Improvement Programme. This major component of SASRI activities consists of five primary areas of research and operations, namely; crossing, selection, genotype testing, bulking and variety release.
Together, this highly co-ordinated series of activities results in the release to the industry of varieties with high sugar yield (both sucrose and cane yield), pest and disease resistance, adaptability, ratooning ability and agronomic and milling characteristics that are desirable to both millers and growers.
Articulating strongly with Commercial Breeding, Variety Characterisation research and development aims to provide comprehensive information on the performance of new varieties under different management practices and agro-climatic conditions upon, or soon after, their release to the industry.
As for Commercial Breeding, Variety Characterisation is complemented by a series of research projects that are instituted to address specific issues, for example variety ratoon longevity and the performance of varieties derived from the NovaCane® Technology.
Introgression Breeding seeks to expand the genetic base of parental germplasm used in Commercial Breeding by performing successive crosses between elite commercial varieties and either plant species closely related to sugarcane or ancestral species that gave rise to modern commercial hybrid varieties. Of particular relevance is that Introgression Breeding provides access to genes from other species that have the potential to contribute improved vigour, resilience and pest and disease resistance to commercial varieties bred through Commercial Breeding.
Research in this area also focuses on the development of methods and technologies to enable the crossing of sugarcane varieties with closely related and progenitor cane species, which includes synchronisation of flowering, pollen storage and genetic diversity analysis.
The SASRI Herbarium is a specialist collection of over 75 dried specimens representing genera closely related to sugarcane with a living collection of 2 659 sugarcane cultivars and sugarcane relatives, focusing on the Saccharinae. An extended living collection of over 300 species native to or naturalised in KwaZulu-Natal, South Africa is also available on the campus. We are estimating around 100 species of grasses on the site, which will be surveyed during flowering season. The herbarium has identified two new species, namely, Tripidium demetae and Saccharum cultum. These are contained both in the living collection and type specimen are also contained within the herbarium collection.
Research and development underlying Trait Development are strongly focused on the development of innovations of strategic importance to the future delivery and sustainable cultivation of varieties with novel traits (characteristics) within the industry.
Traits currently under development include stem borer resistance as well as herbicide and drought tolerance. Research in this area includes the development of technologies and resources required for genetic engineering, mutagenic breeding and the in vitro storage and preservation of valuable germplasm, as well as demonstrating proof-of-concept regarding the performance of the novel lines produced.
While South Africa has a strong regulatory system to oversee agricultural technology, industry stewardship is an essential element in a partnership between government and the private sector. SASRI comply with science-based regulations, demonstrate responsible use and accountability and adhere to biotechnology stewardship principles through an international organization, Excellence Through Stewardship.
https://www.excellencethroughstewardship.org/

This research area focuses on unravelling the extremely complex sugarcane genome with a view to the development of genetic markers linked to important sugarcane traits, for example pest and disease resistance. Knowledge and resources generated in this area feed into breeding research.
Current Projects
Crossing and breeding for agroclimatic zones in southern Africa
Variety evaluation of N cultivars on grower’s farms
Introgression breeding (pre-breeding)
Commercial development of genetically modified (GM) sugarcane for insect borer resistance and herbicide tolerance
Preparative research for regulatory biosafety aspects related to GM commercial release
Investigating alternative Bacillus thuringiensis (Bt) insecticidal proteins for control of lepidopteran borers, yellow sugarcane aphid and thrips
Bioinformatics pipeline development to analyse genomics and transcriptomic data
Identifying defence genes associated with eldana resistance
Mutation breeding for abiotic stress tolerance
Characterisation of stress resistant sugarcane mutants (e.g. for drought , aluminium and salt tolerance)
Long-term storage of valuable germplasm using in vitro techniques including minimal growth and cryopreservation

